Background: Treatment options are limited for patients (pts) with advanced myeloproliferative neoplasms (MPNs). Most pts become refractory to JAK2 inhibitor (JAKi) therapy by 3 years with subsequent survival of only 11-16 months.
Sequential treatment with chromatin-modifying agents including decitabine (D) can upregulate CXCR4 expression, correct abnormal trafficking of myelofibrosis (MF) stem cells, and eliminate JAK2V617F+ MF stem and progenitor cells in preclinical models ( Wang et al, Cancer Res 2019, Blood 2010). Combination therapy with intravenous D and ruxolitinib (Rux) had a favorable effect on overall survival (OS) in a phase 2 study of MPN-accelerated/blast phase pts ( Mascarenhas et al, Blood Adv 2020) and prolonged survival in a cohort of DIPSS-plus high-risk MF pts ( Bader et al, Leuk Res 2015). Oral decitabine/cedazuridine (DC), therefore, represents an attractive ambulatory treatment option for pts with advanced MPNs.
Methods: We retrospectively reviewed the electronic health records of pts with advanced high-risk MF (AHR-MF) or MPN- accelerated phase (MPN-AP) seen at our institution that were treated with DC with or without a JAKi from 1/2021-7/2023. MPN-AP was defined by the presence of 10-19% blasts in peripheral blood/ bone marrow. AHR-MF was defined by the presence of 4-9% circulating blasts or 5-9% blasts in the bone marrow, and/or refractoriness to a JAKi.
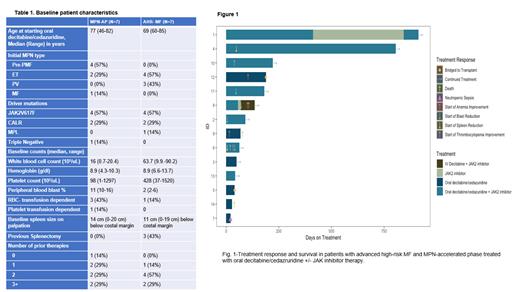
Results: A total of 14 pts, 7 each with MPN-AP and AHR-MF, respectively received DC therapy (Table 1). Most pts (8/14 or 57%) were older than 75 years. All pts were classified as high risk/very high-risk (MIPPS70 plus v.2). Ten (71%) pts had one or more high molecular risk mutations ( ASXL1, EZH2, SRSF2, IDH1/2, U2AF1), with the most prevalent being ASXL1 mutations in 8/14 (57%) pts. Other somatic mutations included NF1, KRAS, NRAS, BRAF, GATA2, STAG2, DNMT3A and TET2 genes. Eight of the 14 pts (57%) had either an unfavorable or very high-risk karyotype (MIPSS70 plus v.2). Thirteen pts had previously failed JAKi therapy. The median number of cycles of DC therapy was 2 (range,1-6) and 4 (range,1-26) for MPN-AP and AHR-MF pts, respectively and 4/7 pts received a concurrent JAKi in each group. Two pts in AHR-MF cohort were successfully bridged to transplant. Two pts in the MPN-AP group discontinued treatment after cycle 1 due to neutropenic sepsis and progression to blast phase, respectively.
Among MPN-AP pts who received ≥ 2 cycles of DC, circulating blasts were reduced to ≤ 5% in 4/5 pts, normalization of thrombocytopenia and improvement in hemoglobin by ≥ 1.5 g/dl each occurred in 3/5 pts (Fig.1). In the AHR-MF cohort, 6/7 pts received ≥ 2 cycles of DC with elimination of circulating blasts in one pt. WBC and platelet count were effectively reduced in 4/4 and 2/2 pts with baseline WBC of >50x 10 3/uL and platelet count >1000 x 10 3/uL, respectively. Spleen size reduction was achieved in two pts with combination of DC + Rux who were previously refractory to a JAKi. One pt achieved complete resolution of palpable splenomegaly after 2 cycles of combination therapy.
The predominant treatment-related adverse event was neutropenic fever in three pts requiring hospitalization. DC cycle length was reduced from 5 to 3/28 days in 7/14 pts, which reduced the degree of therapy related neutropenia and anemia. Pts remained ambulatory during treatment except for hospitalizations related to neutropenic fever. With a median follow-up of 109 days (range, 28 days - 29 months), median OS has not been reached. Three pts in the MPN-AP cohort progressed to MPN-blast phase (BP), including two pts who only received one cycle of DC. Two pts with MPN-BP died after 28 and 68 days of DC therapy, respectively. None of the AHR-MF pts progressed to MPN-AP/BP. The most durable response was observed in a pt who has received a total of 26 cycles of DC + Rux with stable disease for >2 years and loss of BRAF and KRAS mutations after 9 months of treatment.
Conclusions: DC therapy appears to be a well-tolerated and effective oral ambulatory regimen with clinically meaningful responses in elderly, transplant ineligible pts as well as a bridge to transplant in high-risk pts with advanced MPNs. The ease of outpatient administration significantly reduces pt treatment burdens and positively impacts their quality of life. These data provide a rationale for studying oral decitabine/cedazuridine +/- JAKi in a prospective manner, especially in pts with AHR-MF refractory to JAKi prior to evolution MPN-AP/BP.
OffLabel Disclosure:
Tremblay:Sierra Oncology: Consultancy; GSK: Consultancy; Cogent Biosciences: Consultancy; Astellas Pharma: Research Funding; Gilead: Research Funding; AbbVie: Consultancy; Novartis: Consultancy; CTI Biopharma: Consultancy, Research Funding. Mascarenhas:Bristol Myers Squibb, Celgene, Constellation Pharmaceuticals/MorphoSys, CTI BioPharma, Galecto, Geron, GSK, Incyte Corporation, Karyopharm Therapeutics, Novartis, PharmaEssentia, Prelude Therapeutics, Pfizer, Merck, Roche, AbbVie, Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie, Bristol Myers Squibb, Celgene, CTI BioPharma, Geron, Incyte Corporation, Novartis, Janssen, Kartos Therapeutics, Merck, PharmaEssentia, Roche: Research Funding; Incyte, Novartis, Roche, Geron, GSK, Celgene/BMS, Kartos, AbbVie, Karyopharm, PharmaEssentia, Galecto, Imago, Sierra Oncology, Pfizer, MorphoSys, CTI Bio: Consultancy; Bristol Myers Squibb, Celgene, CTI BioPharma, Geron, Incyte Corporation, Janssen, Kartos Therapeutics, Merck, Novartis, PharmaEssentia, Roche; Participated in consulting or advisory committees - AbbVie, Bristol Myers Squibb, Celgene, Constellation Pharmac: Research Funding; AbbVie, CTI BioPharma Corp, a Sobi company, Geron, GlaxoSmithKline, Imago, Incyte, Kartos, Kayropharm, MorphoSys, Novartis, Pfizer, PharmaEssentia, Sierra: Consultancy; GSK: Honoraria. Kremyanskaya:Protagonist Therapeutics, Inc.: Consultancy, Research Funding. Ginzburg:Protagonist Therapeutics, Inc.: Consultancy, Research Funding. Hoffman:Karyopharm: Research Funding; Kartos Abbvie: Research Funding; Curis: Research Funding; Dexcel Pharma: Research Funding; Summitomo: Research Funding; Dompe: Patents & Royalties; Silence Therapeutics: Consultancy; TD2: Research Funding; Cellinkos: Consultancy; Protagonist Therapeutics: Consultancy.
Oral decitabine-cedazuridine is FDA approved for previously treated and untreated, intermediate-high risk, de novo and secondary MDS and CMML. We report on its off-label use in advanced myeloproliferative neoplasms including MPN-accelerated phase.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal